Topic: SEPARATION (Kuparadzanisa Zvinhu)
Hello Students!
Sometimes we have mixtures of different things mixed together. For example, ink (ingi) might look like one colour, but it's often a mixture of several different coloured dyes. How can we separate these colours to see what's inside? One simple way is called Paper Chromatography. "Chroma" means colour, and "graphy" means writing or drawing – so it's like "colour writing"!
Chromatography (Kuparadzanisa nePaper)
Describing Paper Chromatography (What it is and How it Works)
- What is it? Paper chromatography is a method used to separate dissolved substances (things that can melt into a liquid, like salt in water or dyes in ink) from a mixture. It works especially well for separating coloured substances like inks or plant pigments (colours from leaves).
- (zvinrehwa apa ndezvekuti kana uka mixer zvinhu zvakaita mvura mvura or ma colors aya epashizha akana pahembe dzako, unokwanisa ku separater amcolors iwayo, lets say ukungoona hembe yako ndeye blue, kuti blue iyoyo ivepopakaiswama dye anema colour akasiyana siyana, tokwanisa kutoona hembe iyoyo kuti yakagadzirwa nema colour api ne api)
- How does it work? It works because different substances travel at different speeds through a special piece of paper when carried along by a liquid (called a solvent). (izvi zvinoitika nekuti zvinhu zvinogadzira ma coloursiwayo tikazviisa pa bepa iri rinonzi Chroma tikadira tumvura tunonz a solvent zvinotanga ku speader out, saka paku spreader out pa zvinomhanya ne speed dzakasiyana saka pazhinozomira totoona for example blue yatoo kure ichiteverwane green ichiteverwa ne yellow.)

What You Need (Materials):
- Chromatography Paper: A special type of paper that absorbs liquid well (like filter paper or even paper towel sometimes works for simple tests). This paper is the stationary phase (it stays still).
- Solvent: A liquid that can dissolve the mixture you want to separate (e.g., water, alcohol/spirit, or other special liquids). The solvent is the mobile phase (it moves).
- The Mixture: The substance you want to separate (e.g., a spot of black ink).
- A Container: Like a beaker or jar, with a lid if possible.
Steps (How to do it):
- Draw a Line: Take a strip or square of chromatography paper. Use a pencil (penzura) to draw a line near the bottom edge (maybe 1-2 cm up). Use pencil because pencil lead doesn't dissolve in the solvent and won't move.
- Spot the Mixture: Put a small spot of your mixture (e.g., black ink) right in the middle of the pencil line. Let it dry. You might put another spot on top to make it stronger.
- Prepare the Container: Pour a small amount of the solvent into the container. The level should be lower than the pencil line on your paper.
- Place the Paper In: Carefully hang or stand the paper strip in the container so that the bottom edge (below the spot) dips into the solvent. Make sure the ink spot itself does NOT touch the solvent directly.
- Wait and Watch: Cover the container (if you have a lid) to stop the solvent from evaporating too quickly. Watch as the solvent slowly moves up the paper by soaking into it (this is called capillary action).
- Separation Happens: As the solvent front moves up past the ink spot, it dissolves the different coloured dyes in the ink.
- Different Speeds: The dyes travel up the paper with the solvent, BUT they travel at different speeds:
- Dyes that dissolve easily in the solvent AND don't stick strongly to the paper travel faster and further up the paper.
- Dyes that don't dissolve as well OR stick strongly to the paper travel slower and not as far up the paper.
- Stop and Dry: When the solvent has moved almost to the top of the paper, take the paper out of the container. Mark the highest point the solvent reached (the solvent front) with a pencil quickly before it dries. Let the paper dry completely.
- The Result (Chromatogram): You will see that the original single ink spot has separated into several spots of different colours at different heights on the paper. This pattern of separated spots is called a chromatogram.
Applications of Paper Chromatography (Uses)
Why is this simple technique useful? It has many practical uses:
- Separating Inks and Dyes:
- To see what colours are mixed together in a particular ink (like in the experiment above).
- In forensic science (police work) to compare ink found at a crime scene (like on a threatening note) with ink from a suspect's pen.
- Checking Food Colourings:
- To identify the different artificial colours added to sweets, drinks, or processed foods.
- To check if the food colourings used are safe and allowed by law.
- Identifying Substances:
- To separate and identify pigments (colours) from plants (like chlorophyll from leaves).
- To detect certain substances like amino acids (building blocks of proteins) or sugars.
- Sometimes used to detect drugs or poisons in samples (though other methods are often more precise).
- Checking Purity:
- If a substance is pure, it should ideally only give one spot on the chromatogram (using a suitable solvent). If you see multiple spots, it means the substance is impure (it's a mixture).
Key Idea: Paper chromatography is a simple, cheap, and effective way to separate small amounts of dissolved substances in a mixture, based on how differently they move up paper with a solvent.
Let's Summarise: Chromatography
- Paper Chromatography separates dissolved substances in a mixture.
- It uses paper (stationary phase) and a solvent (mobile phase).
- Substances separate because they travel at different speeds based on how well they dissolve in the solvent and stick to the paper.
- The result is a pattern of spots called a chromatogram.
- It is used to separate inks, check food colours, identify substances, and check purity.
Topic: MATTER - Elements and Reactions
Hello Students!
Everything around us is made of matter. Matter is made of tiny building blocks called atoms. Different types of atoms make different elements. Scientists arrange all the known elements in a special chart called the Periodic Table. Elements in the same column (called a Group) often behave in similar ways because they have a similar arrangement of their tiny parts (electrons) on the outside.
Today we look at Groups I, II, VII, and VIII, and how metals react.
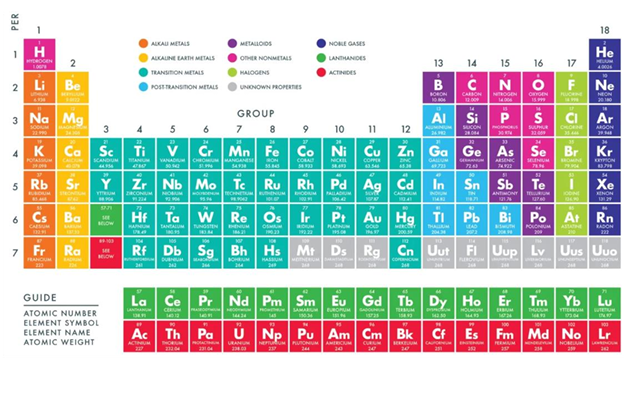
Properties of Specific Groups in the Periodic Table
A. Group I: The Alkali Metals
Who they are: Lithium (Li), Sodium (Na), Potassium (K), etc. (Found in the very first column on the left).
Properties:
- Soft Metals: You can usually cut them easily with a knife (especially Na and K).
- Low Density: Some (Li, Na, K) are light enough to float on water.
- Shiny when cut: But they quickly become dull because they react with air (mweya).
- VERY REACTIVE: They react strongly and quickly with water (mvura), oxygen (in the air), and Group VII elements. They really want to join up with other elements!
- One Outer Electron: They have only 1 electron (tiny particle) in their outermost layer, which they lose easily when they react.
- Reactivity increases down the group: Potassium (K) is more reactive than Sodium (Na), which is more reactive than Lithium (Li).
B. Group II: The Alkaline Earth Metals
Who they are: Beryllium (Be), Magnesium (Mg), Calcium (Ca), etc. (Found in the second column from the left).
Properties:
- Metals: Harder than Group I metals.
- Higher Melting Points than Group I.
- Shiny: But also react with air to become dull over time.
- REACTIVE: But generally less reactive than Group I metals. They react with water (Ca reacts well, Mg reacts with hot water/steam), oxygen (especially when heated), and acids.
- Two Outer Electrons: They have 2 electrons in their outermost layer, which they lose when they react.
- Reactivity increases down the group: Calcium (Ca) is more reactive than Magnesium (Mg).
C. Group VII: The Halogens
Who they are: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I). (Found in the second column from the right).
Properties:
- Non-Metals: They are not metals.
- Coloured: Fluorine (pale yellow gas), Chlorine (greenish-yellow gas), Bromine (red-brown liquid), Iodine (grey-black solid that turns into purple vapour when heated).
- Diatomic Molecules: They exist as pairs of atoms joined together (F₂, Cl₂, Br₂, I₂).
- VERY REACTIVE Non-Metals: They react easily with metals to form salts (munyu). (e.g., Sodium + Chlorine -> Sodium Chloride, which is common table salt).
- Seven Outer Electrons: They have 7 electrons in their outermost layer and strongly want to gain 1 more electron when they react.
- Reactivity decreases down the group: Fluorine (F) is the most reactive, Iodine (I) is the least reactive among these.
D. Group VIII: The Noble Gases
Who they are: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), etc. (Found in the last column on the far right).
Properties:
- Gases: All are gases at room temperature.
- Colourless and Odourless.
- VERY UNREACTIVE (Inert): They almost never react with other elements. Why? Because they have a full outer shell of electrons, making them very stable and "happy" on their own.
- Exist as Single Atoms: They don't join up with each other or other elements easily.
Uses of Halogens (Group VII)
Because they are reactive, halogens have important uses:
- Chlorine (Cl₂):
- Killing Germs: Used to sterilize drinking water and swimming pool water. Kills harmful bacteria.
- Bleach: Used in household bleach to remove colours and kill germs.
- Making Plastics: Used to make PVC plastic (for pipes, window frames).
- Iodine (I₂):
- Antiseptic: Used to clean wounds and prevent infection (like in tincture of iodine - iodhini yekurapa ronda).
- Needed by the Body: A tiny amount is needed for our thyroid gland to work properly (often added to table salt - "iodised salt").
- Fluorine (F - usually as fluoride compounds):
- Toothpaste: Added to toothpaste (as fluoride) to help strengthen teeth and prevent tooth decay.
Reactions of Metals
Metals (simbi) react in different ways depending on what they are reacting with and how reactive the metal itself is.
A. Reaction with Water and Steam:
- Very Reactive Metals (K, Na, Ca): React with cold water.
- Observation: Fizzing (gas produced), metal may melt (Na, K) or move around, solution becomes alkaline (slippery feel). K and Na can even catch fire!
- Products: Metal Hydroxide + Hydrogen gas
- Example (Sodium): Sodium + Water ---> Sodium Hydroxide + Hydrogen
- Less Reactive Metals (Mg, Al, Zn, Fe): Do not react much with cold water, but react with steam (water as a hot gas). Requires heating.
- Observation: Metal may glow, white/grey powder (oxide) forms, gas produced.
- Products: Metal Oxide + Hydrogen gas
- Example (Magnesium): Magnesium + Steam ---> Magnesium Oxide + Hydrogen
- Unreactive Metals (Pb, Cu, Ag, Au): Do not react with cold water or steam.
B. Reaction with Air (Oxygen):
- Most metals react with oxygen when heated to form Metal Oxides.
- Very Reactive Metals (K, Na, Ca, Mg): React very quickly, often burning brightly with a flame.
- Example (Magnesium): Magnesium + Oxygen ---> Magnesium Oxide (bright white flame, white powder forms)
- Less Reactive Metals (Zn, Fe, Pb, Cu): React more slowly when heated, forming an oxide layer on the surface. Iron rusting is a slow reaction with oxygen and water.
- Example (Copper): Copper + Oxygen ---> Copper Oxide (black powder forms when copper is heated strongly)
- Unreactive Metals (Ag, Au, Pt): Do not react easily with oxygen, even when heated. That's why gold stays shiny.
C. Reaction with Dilute Acids (like Hydrochloric Acid - HCl, Sulfuric Acid - H₂SO₄):
- Metals more reactive than Hydrogen react with dilute acids.
- Observation: Fizzing (gas produced), metal dissolves, solution gets warm.
- Products: Salt + Hydrogen gas
- Example (Zinc): Zinc + Hydrochloric Acid ---> Zinc Chloride + Hydrogen
- How fast it reacts depends on the metal's reactivity: Magnesium reacts very fast, Zinc reacts fast, Iron reacts steadily, Lead reacts very slowly.
- Metals less reactive than Hydrogen (Cu, Ag, Au) do not react with dilute acids to produce hydrogen gas. If you put copper in dilute HCl, nothing happens.
Writing Equations for Reactions of Metals
We can show these reactions using words or chemical symbols.
Word Equations:
- Magnesium + Oxygen → Magnesium Oxide
- Zinc + Sulfuric Acid → Zinc Sulfate + Hydrogen
- Calcium + Water → Calcium Hydroxide + Hydrogen
Symbol Equations (using chemical formulas):
(Note: Balancing equations with numbers is important but might be taught later. These show the substances involved.)
- Metal + Oxygen:
2Mg + O₂ → 2MgO
2Cu + O₂ → 2CuO - Metal + Dilute Acid:
Zn + 2HCl → ZnCl₂ + H₂
Fe + H₂SO₄ → FeSO₄ + H₂ - Metal + Water:
2Na + 2H₂O → 2NaOH + H₂ (Reaction with cold water)
Mg + H₂O(steam) → MgO + H₂ (Reaction with steam)
The Reactivity Series of Metals
- Scientists have found that some metals are much more reactive than others. They arranged the common metals in a list called the Reactivity Series, from most reactive at the top to least reactive at the bottom.
- List (decreasing reactivity - most reactive first):
Please Stop Calling Me A Careless Zebra Instead Try Learning How Copper Saves Gold (& Platinum)
(This sentence can help remember the order!)- Potassium (K) - Most Reactive
- Sodium (Na)
- Calcium (Ca)
- Magnesium (Mg)
- Aluminium (Al)
- (Carbon (C)) - (Non-metal, included for comparison)
- Zinc (Zn)
- Iron (Fe)
- Tin (Sn) - (Sometimes included)
- Lead (Pb)
- (Hydrogen (H)) - (Non-metal, included for comparison)
- Copper (Cu)
- Silver (Ag)
- Gold (Au)
- Platinum (Pt) - Least Reactive
- Why include Carbon and Hydrogen? They are useful reference points. Metals above Carbon can usually be extracted using carbon. Metals above Hydrogen react with dilute acids to give off hydrogen gas.
Predicting Reactivity from the Series
The Reactivity Series helps us predict how metals will behave:
- Reaction Intensity: Metals higher up react more vigorously (faster, more heat) with water, acids, or oxygen than metals lower down.
- Example: Calcium reacts much faster with water than Magnesium does (because Ca is higher than Mg).
- Reaction with Water/Acid: Metals above Hydrogen will react with dilute acids to produce hydrogen gas. Metals below Hydrogen will not.
- Displacement Reactions: A more reactive metal can push (displace) a less reactive metal out of its compound (usually in a solution).
- Example: Zinc is above Copper. If you put a piece of Zinc metal into a blue solution of Copper Sulfate:
Zinc + Copper Sulfate → Zinc Sulfate + Copper
The blue colour fades (as copper sulfate is used up), and reddish-brown copper metal appears. Zinc has pushed copper out. - Example: Copper is below Zinc. If you put Copper metal into Zinc Sulfate solution, nothing happens because Copper is less reactive and cannot push Zinc out.
- Example: Zinc is above Copper. If you put a piece of Zinc metal into a blue solution of Copper Sulfate:
Let's Summarise: Elements & Reactions
- Elements in Group I (soft, very reactive metals), Group II (reactive metals), Group VII (reactive non-metals - Halogens), and Group VIII (unreactive Noble gases) have distinct properties based on their position in the Periodic Table.
- Halogens are used for disinfection (Chlorine) and antiseptics (Iodine).
- Metals react with water/steam, air (oxygen), and dilute acids depending on their reactivity. Products are usually metal hydroxides/oxides and hydrogen, or salts and hydrogen.
- The Reactivity Series lists metals from most reactive (Potassium) to least reactive (Platinum).
- We can use the series to predict how strongly a metal will react and whether it can displace another metal from its compound.
Topic: ACIDS, BASES, AND SALTS (Zvinhu Zvinovava, Zvinotsvedza, neMunyu)
Sub-Topic: Acid-Base Titration (Kuyera Simba reAcid kana Base)
Hello Students!
We know about acids (like lemon juice, vinegar, or battery acid - asidhi) and bases (also called alkalis if they dissolve in water - like soap, ash water, or ammonia solution - besi/arakari). Acids and bases react together in a process called neutralization.
Sometimes, we know the exact strength (concentration) of an acid, but we don't know the strength of a base. Or maybe we know the base's strength but not the acid's. Titration is a very careful experiment we do in the lab to find out the unknown strength (concentration) of an acid or a base using one whose strength we do know accurately.
Apparatus Used in Titration (Midziyo Inoshandiswa)
To do titration properly, we need some special equipment:
- Burette (sound: Bhiyureti):

- What it is: A long, thin glass tube with markings (a scale) along the side to measure volume accurately. It has a tap (tapi) at the bottom to control the flow of liquid.
- Its Job: To deliver variable but precisely measured volumes of one solution (e.g., the acid).
- Pipette (sound: Paipeti):

- What it is: A glass tube designed to measure one fixed, very accurate volume of liquid (e.g., 20 cm³ or 25 cm³). It often has a bulge in the middle and a mark on the narrow neck.
- Its Job: To transfer an exact starting volume of the other solution (e.g., the base) into the flask.
- (Pipette Filler): Often used with a pipette for safety (instead of using your mouth) to suck the liquid up.
- Conical Flask :

- What it is: A flask with a flat bottom and cone-shaped sides, narrowing towards the top.
- Its Job: To hold the solution measured by the pipette. The shape allows you to swirl the liquid easily without spilling it.
- Indicator :
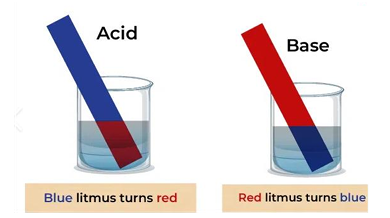
- What it is: A special chemical dye that changes colour when the solution changes from acidic to alkaline, or vice versa.
- Its Job: To signal the exact point when the acid and base have completely reacted (neutralized each other). This point is called the endpoint.
- Examples: Litmus, Phenolphthalein (pink in alkali, colourless in acid), Methyl Orange (yellow in alkali, red in acid).
- White Tile (Tairosi Jena):

- What it is: A flat piece of white ceramic tile.
- Its Job: Placed under the conical flask, it makes it much easier to see the indicator's colour change clearly.
- Clamp Stand (or Retort Stand) (Standhi neClamp):

- What it is: A heavy base with a vertical rod and adjustable clamps.
- Its Job: To hold the burette securely upright during the experiment.
- Funnel (Fanera):

- What it is: A cone-shaped tool used for pouring liquids into narrow openings.
- Its Job: To help fill the burette safely without spilling.
Acid-Base Titration Procedure (Maitirwo eTitration)
Here are the steps for a typical titration, for example, finding the concentration of an acid using a base of known concentration:
Preparation:
- Rinse Equipment: Rinse the burette with a small amount of the acid solution it will contain. Rinse the pipette with a small amount of the base solution it will measure. This removes any water drops that would change the concentration.
- Set up Burette: Clamp the burette vertically in the stand. Close the tap. Use a funnel to carefully fill the burette with the acid solution. Fill it slightly above the zero mark.
- Remove Air Bubble & Set Zero: Remove the funnel. Open the tap briefly to run out some acid, ensuring the tip below the tap is full of acid (no air bubble). Read the starting volume at the bottom of the curved surface (meniscus) of the liquid – adjust until it is exactly on the 0.00 cm³ mark or record the exact starting reading.
- Prepare Flask: Use the pipette (with filler) to measure an exact volume (e.g., 25.0 cm³) of the base solution into the clean conical flask.
- Add Indicator: Add 2 or 3 drops of a suitable indicator (e.g., phenolphthalein) to the base in the flask. Note the starting colour (it will be pink if using phenolphthalein with a base). Place the flask on the white tile directly under the burette tip.
Performing the Titration:
- Rough Titration (First Try): Open the burette tap and add the acid to the flask fairly quickly at first, while constantly swirling the flask to mix the solutions. Watch the colour. As the acid is added, the pink colour might disappear where the acid drops in but reappear on swirling. Slow down when the colour change starts to last longer. Add the acid drop by drop near the end until one drop causes the indicator to change colour permanently (e.g., pink just disappears completely for phenolphthalein). This is the endpoint.
- Record Volume: Read the final volume on the burette accurately (to two decimal places, e.g., 22.50 cm³). Calculate the volume of acid used (Final reading - Initial reading). This first result is usually a rough estimate.
- Accurate Titrations (Repeat):
- Rinse the conical flask well with water (or use a clean one).
- Refill the burette if needed (record new starting volume).
- Pipette another exact volume of base into the flask, add indicator.
- Now, titrate carefully. Add the acid quickly at first until you get close to the rough volume (e.g., until about 21 cm³ if the rough was 22.50 cm³).
- Then add the acid drop by drop, swirling after each drop, until the endpoint is reached exactly (the very first permanent colour change).
- Record the final burette reading and calculate the volume used.
- Repeat Again: Repeat the accurate titration (step 3 above - item 8 in original) at least two more times. You want to get results that are very close to each other (usually within 0.10 cm³ or 0.20 cm³). These are called concordant results.
Using the Results:
- Calculate Average: Use the concordant results (ignore the rough one if it's very different) to calculate the average volume of acid used.
- Calculation: This average volume is then used in calculations (often involving formulas) along with the known concentration of the base and the volumes used, to find the unknown concentration of the acid.
Carrying Out Acid-Base Titration (Practical Notes)
- Accuracy is Key: Read the burette carefully at eye level to avoid parallax error. Ensure the pipette volume is exact. Stop at the very first sign of a permanent colour change.
- Swirling: Swirl the flask constantly, especially near the endpoint, to make sure the acid and base mix properly.
- Safety: Wear safety glasses (magirazi ekudzivirira maziso)! Acids and bases can be corrosive. Use a pipette filler, not your mouth. Clean up any spills immediately.
- Consistency: Use the same indicator and the same number of drops for each titration.
Titration requires patience and careful measurement, but it's a fundamental skill in chemistry for finding out exactly "how much" of a substance is present in a solution.
Let's Summarise: Titration
- Titration is a method to find the concentration of an acid or base using a known concentration of the other.
- Key apparatus includes: Burette, Pipette, Conical Flask, Indicator, White Tile, Clamp Stand.
- The procedure involves carefully adding one solution from the burette to an exact volume of the other solution (with indicator) in the flask, until the indicator changes colour permanently (endpoint).
- The experiment is repeated several times to get accurate and reliable (concordant) results.
- The volume measured from the burette (titre) is used to calculate the unknown concentration.
Topic: INDUSTRIAL PROCESSES
Hello Students!
Factories use chemistry on a large scale to make useful products. Today we'll learn about two very important processes: The Haber Process for making ammonia, and the Contact Process for making sulfuric acid.
Sub-Topic 1: The Haber Process - Making Ammonia (NH₃)
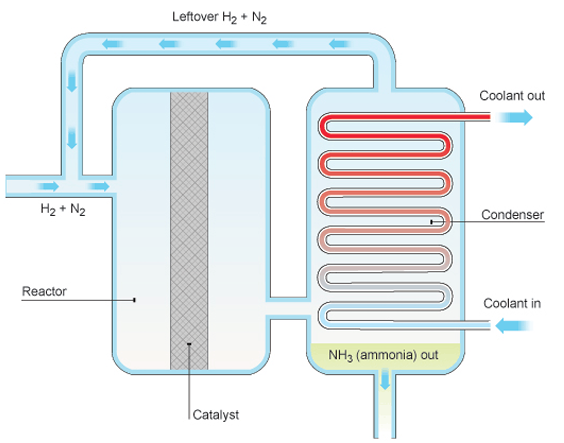
Ammonia is a very important chemical, mostly used to make fertilizers (manyowa) that help crops grow better.
1. Raw Materials Used (Zvinhu Zvinoshandiswa Kutanga Nazvo):
Where do we get the ingredients for ammonia (which has the formula NH₃)?
- Nitrogen (N₂): Comes from the Air. Air is about 78% nitrogen gas. Factories separate it from the other gases in the air (like oxygen).
- Hydrogen (H₂): Usually comes from reacting Natural Gas (mainly methane, CH₄) with steam (hot water vapour). Sometimes it's made from water using electricity (electrolysis).
2. Description of the Manufacture (Maitirwo Acho):
Making ammonia involves reacting Nitrogen gas and Hydrogen gas together.
- The Reaction: Nitrogen gas reacts with Hydrogen gas to form Ammonia gas. This reaction can also go backwards (ammonia can break down into nitrogen and hydrogen), so we write it with a special arrow:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g) (g = gas; ⇌ means reversible) - Steps:
- Mix the purified Nitrogen and Hydrogen gases in the correct ratio (1 part Nitrogen to 3 parts Hydrogen by volume).
- Compress the gas mixture to a very high pressure.
- Heat the mixture to a high temperature.
- Pass the hot, high-pressure gases over a catalyst (a substance that speeds up the reaction without being used up itself). The catalyst used here is Iron.
- Some of the nitrogen and hydrogen react to form ammonia gas. Because the reaction is reversible, not all of it reacts at once.
- Cool the mixture of gases leaving the catalyst chamber. Ammonia gas turns into a liquid at a higher temperature than nitrogen and hydrogen.
- Collect the liquid ammonia.
- The unreacted nitrogen and hydrogen gases are recycled – sent back to the catalyst chamber to react again. This saves resources and makes the process efficient.
3. Conditions Needed (Mamiriro Anodiwa):
To make the reaction happen efficiently (fast enough and producing a good amount of ammonia), specific conditions are needed:
- Temperature: About 400 - 450 °C. This is hot, but not too hot. Higher temperatures make the reaction faster, but also favour the backward reaction (breaking down ammonia). So, this is a compromise temperature.
- Pressure: Very High Pressure, about 200 atmospheres (atm) (200 times normal air pressure). High pressure pushes the gas molecules closer together and favours the forward reaction (making ammonia, because 4 gas molecules turn into only 2 gas molecules).
- Catalyst: Iron catalyst. This speeds up both the forward and backward reactions, allowing the compromise temperature to work well enough.
4. Industrial Uses of Ammonia (Mashandisiro eAmmonia):
Why make so much ammonia?
- Fertilizers: This is the biggest use! Ammonia is used to make ammonium nitrate, ammonium sulfate, and urea, which are put on fields to give plants the nitrogen they need to grow strong.
- Making Nitric Acid: Ammonia is reacted with oxygen to make nitric acid, another important industrial chemical.
- Making Nylon: Used in the process of making nylon fibres (for clothes, ropes, carpets).
- Refrigeration: Ammonia gas was used as a refrigerant (cooling agent) in large refrigerators and factories (though less common now in homes).
- Cleaning Products: Used in some household cleaners.
- Explosives: Nitric acid (made from ammonia) is used to make explosives.
Sub-Topic 2: The Contact Process - Making Sulfuric Acid (H₂SO₄)
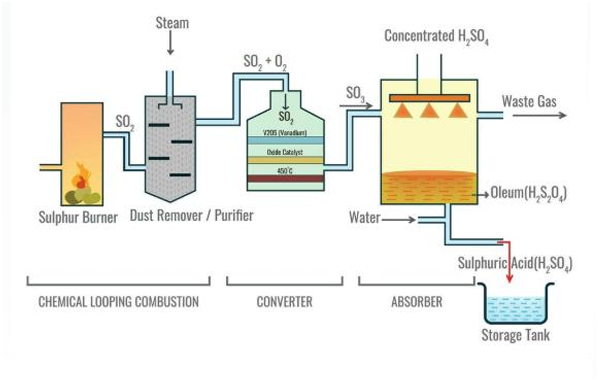
Sulfuric acid is one of the most widely used chemicals in industry. It's a very strong acid.
1. Raw Materials Used (Zvinhu Zvinoshandiswa Kutanga Nazvo):
Where do we get the ingredients for sulfuric acid (H₂SO₄)?
- Sulfur (S): Can be mined directly from the ground as yellow solid sulfur. Can also be obtained by removing sulfur from fossil fuels (like oil and natural gas) or from roasting metal sulfide ores (like pyrite - iron sulfide).
- Air: Provides the Oxygen (O₂) needed.
- Water (H₂O): Needed in the final step.
2. Description of the Manufacture (Maitirwo Acho):
Making sulfuric acid is a multi-step process.
- Step 1: Make Sulfur Dioxide (SO₂):
- Burn sulfur powder or molten sulfur in a good supply of dry air.
- S(s) + O₂(g) ---> SO₂(g) (s = solid, g = gas)
- (If starting from sulfide ores, roasting the ore also produces SO₂).
- Step 2: Make Sulfur Trioxide (SO₃) - The "Contact" Step:
- Mix the sulfur dioxide gas with more dry air (oxygen).
- Pass this mixture over a catalyst at the right temperature. This reaction is reversible.
- 2SO₂(g) + O₂(g) ⇌ 2SO₃(g)
- Step 3: Make Sulfuric Acid (H₂SO₄):
- You cannot just add sulfur trioxide (SO₃) directly to water because it reacts violently, creating a dangerous mist of acid.
- Instead, dissolve the sulfur trioxide gas in concentrated sulfuric acid that has already been made. This forms a thick, oily liquid called Oleum (H₂S₂O₇).
- SO₃(g) + H₂SO₄(l) ---> H₂S₂O₇(l) (l = liquid)
- Then, carefully mix the Oleum with the correct amount of water to produce concentrated sulfuric acid (usually about 98% pure).
- H₂S₂O₇(l) + H₂O(l) ---> 2H₂SO₄(l)
3. Conditions Needed (Mamiriro Anodiwa - especially for Step 2):
The key step is converting SO₂ to SO₃. Conditions are chosen for efficiency:
- Temperature: About 450 °C. This is a compromise temperature (similar reasons as Haber process).
- Pressure: About 1 - 2 atmospheres (atm) (only slightly above normal air pressure). High pressure helps a bit, but the reaction works well enough at low pressure, which is cheaper to build and run.
- Catalyst: Vanadium(V) Oxide (V₂O₅). This catalyst speeds up the reaction SO₂ + O₂ ⇌ SO₃ effectively at the chosen temperature.
4. Uses of Sulfuric Acid (Mashandisiro eSulfuric Acid):
Sulfuric acid is used in making many, many things:
- Fertilizers: Making phosphate fertilizers like superphosphate, and ammonium sulfate. (This is a major use!)
- Detergents: Used in making soaps and washing powders (sipo yekuwachisa).
- Paints and Pigments: Used in making coloured paints.
- Fibres: Making fibres like rayon.
- Cleaning Metals (Pickling): Used to remove rust and scale from steel before it's coated (e.g., galvanized).
- Car Batteries (bhatiri remota): The liquid inside lead-acid car batteries is dilute sulfuric acid.
- Oil Refining: Used in processing crude oil.
- Making Other Chemicals: Used as a catalyst or reactant in countless other chemical processes.
Let's Summarise: Industrial Processes
- The Haber Process makes Ammonia (NH₃) from Nitrogen (from air) and Hydrogen (from natural gas), using high temperature (450°C), high pressure (200 atm), and an Iron catalyst. Ammonia is mostly used for fertilizers.
- The Contact Process makes Sulfuric Acid (H₂SO₄) from Sulfur, Air (Oxygen), and Water, in several steps. The key step (SO₂ to SO₃) uses medium temperature (450°C), low pressure (1-2 atm), and a Vanadium(V) Oxide catalyst. Sulfuric acid is used for fertilizers, detergents, paints, batteries, and many other things.
Topic: OXIDATION AND REDUCTION & IRON ALLOYS
Hello Students!
Today we are going to learn about two connected types of chemical reactions called Oxidation and Reduction. These reactions are happening all around us, like when iron rusts or when we burn fuel. Then, we'll look at iron (simbi yesando) and how mixing it with small amounts of other elements can make it stronger or stop it from rusting.
Oxidation and Reduction (also known as Redox Reactions)
These two terms describe processes that often happen together in a chemical reaction. If one thing is oxidized, another thing must be reduced at the same time. We call reactions where this happens Redox reactions.
There are a few ways to think about oxidation and reduction:
A. In Terms of Oxygen:
- Oxidation: Is the GAIN of Oxygen by a substance.
- Example: When magnesium burns in air, it gains oxygen to form magnesium oxide. Magnesium + Oxygen ---> Magnesium Oxide. Here, Magnesium has been oxidized.
- Example: When iron rusts, it combines with oxygen. Iron + Oxygen ---> Iron Oxide (Rust). Iron has been oxidized.
- Reduction: Is the LOSS of Oxygen from a substance.
- Example: When copper oxide reacts with hydrogen, the copper oxide loses its oxygen to become copper metal. Copper Oxide + Hydrogen ---> Copper + Water. Here, Copper Oxide has been reduced.
B. In Terms of Electrons (Tiny negative particles in atoms):
This is often a more useful way to think about it in chemistry.
Remember: OIL RIG
- Oxidation Is Loss (of electrons)
- Reduction Is Gain (of electrons)
- Oxidation: Is the LOSS of Electrons by an atom, molecule, or ion.
- Example: When a sodium atom (Na) reacts, it loses one electron to become a positive ion (Na⁺). Na ---> Na⁺ + e⁻ (e⁻ represents an electron). Sodium has been oxidized.
- Reduction: Is the GAIN of Electrons by an atom, molecule, or ion.
- Example: When a chlorine atom (Cl) reacts, it often gains one electron to become a negative ion (Cl⁻). Cl + e⁻ ---> Cl⁻. Chlorine has been reduced.
Why they happen together (Redox):
Electrons don't just disappear or appear from nowhere. If one substance loses electrons (oxidation), another substance must gain those electrons (reduction) in the same reaction.
- Example: When sodium reacts with chlorine: 2Na + Cl₂ ---> 2NaCl (Sodium Chloride - salt)
- Sodium (Na) loses electrons (gets oxidized) to become Na⁺.
- Chlorine (Cl₂) gains electrons (gets reduced) to become Cl⁻ ions.
- So, sodium is oxidized, and chlorine is reduced. It's a Redox reaction.
Alloys of Iron
Pure iron is actually quite soft and not very strong. It also rusts easily (oxidation!). To make iron more useful, we mix it with small amounts of other elements (metals or non-metals like carbon). These mixtures are called Alloys. Alloying changes the properties of the metal, often making it harder, stronger, or resistant to rusting.
A. List of Common Iron Alloys:
- Steel
- Stainless Steel
- Cast Iron
B. Composition, Properties, and Uses:
1. Steel
Composition: Mainly Iron (Fe) mixed with a small amount of Carbon (C) (typically less than 1.7%, often much less, like 0.1% to 1.5%). Different amounts of carbon make different types of steel (e.g., mild steel, high-carbon steel).
Properties:
- Much harder and stronger than pure iron.
- Less malleable (harder to bend) than pure iron.
- Strength and hardness increase with carbon content (but can also become more brittle).
- Still rusts (needs protection like paint or coating).
Uses (depending on carbon content):
- Mild Steel (low carbon): Car bodies, ship hulls, bridges, building supports (beams, rods - simbi dzekuvakisa), nails, wire. (Strong but can be shaped).
- High Carbon Steel: Cutting tools (drills, chisels, saws), springs, hammers. (Very hard, holds a sharp edge).
2. Stainless Steel
Composition: Mainly Iron (Fe) mixed with Chromium (Cr) (key ingredient, typically 10.5% or more) and often Nickel (Ni). Also contains a small amount of Carbon.
Properties:
- Main Property: RESISTANT TO RUSTING AND CORROSION. The chromium forms a very thin, invisible, protective layer of chromium oxide on the surface that stops oxygen and water from reaching the iron.
- Hard and strong.
- Shiny, attractive appearance.
- Hygienic (easy to clean).
Uses:
- Cutlery (knives, forks, spoons - mapanga nemaforogo).
- Kitchen sinks, cooking pots (mapoto esimbi).
- Surgical instruments (midziyo yekuchipatara).
- Equipment for chemical plants and food processing.
- Watch straps, decorative items.
3. Cast Iron
Composition: Mainly Iron (Fe) mixed with a higher amount of Carbon (C) than steel (typically 2% - 4%), and often some Silicon (Si).
Properties:
- Very hard and resistant to wear.
- Brittle – it can break easily if dropped or hit sharply (unlike steel which tends to bend first).
- Good casting properties: Melts at a lower temperature than steel and flows well into moulds (maforoma) to make complex shapes.
- Relatively cheap to produce.
- Good at absorbing vibrations.
- Rusts, but often slower than steel due to silicon content.
Uses:
- Engine blocks in cars and heavy machinery.
- Heavy machine bases.
- Some cookware (heavy frying pans, pots).
- Manhole covers (zvifukidziro zvemanhole).
- Drainpipes, radiators (for heating).
- Decorative railings and gates.
Let's Summarise: Redox & Alloys
- Oxidation is the gain of oxygen OR the loss of electrons (OIL).
- Reduction is the loss of oxygen OR the gain of electrons (RIG).
- Oxidation and Reduction happen together in Redox reactions.
- Alloys are mixtures of metals with other elements to improve properties.
- Steel (Iron + low Carbon) is hard and strong, used in construction and tools.
- Stainless Steel (Iron + Chromium + Nickel) is resistant to rust, used for cutlery and sinks.
- Cast Iron (Iron + high Carbon) is hard but brittle, easy to cast into shapes, used for engine blocks and manhole covers.
Topic: ORGANIC CHEMISTRY & GLOBAL WARMING
Hello Students!
Organic chemistry sounds complicated, but it's about the building blocks of life and many useful materials. We'll start with ethanol, a chemical many people know. Then we'll talk about changes happening to our planet's climate.
Sub-Topic 1: Ethanol (An Important Alcohol)
1. Homologous Series of Ethanol:
- Remember we talked about families of chemicals called homologous series? Ethanol belongs to the Alcohols homologous series.
- What are Alcohols? They are organic compounds that contain the -OH functional group (an oxygen atom bonded to a hydrogen atom) attached to a carbon atom.
- All alcohols have similar chemical properties because they all have this -OH group. They have the general formula CnH₂n+₁OH. Ethanol is the alcohol where n=2.
2. Displayed Structural Formula of Ethanol:
The chemical formula for ethanol is C₂H₅OH.
A displayed structural formula shows every atom and every bond between them.
Ethanol has two carbon atoms bonded together. One carbon atom has the -OH group attached. All the other available bonds on the carbon atoms are filled with hydrogen atoms. (Remember: Carbon always makes 4 bonds).
H H
| |
H - C - C - O - H
| |
H H
- You can see the two Carbon (C) atoms.
- You can see the five Hydrogen (H) atoms attached directly to the Carbon atoms.
- You can see the Oxygen (O) atom bonded to one Carbon and one Hydrogen (the -OH group).
3. Production of Concentrated Ethanol:
Getting highly concentrated ethanol usually involves two main steps:
- Step 1: Fermentation (Kuvirisa):
- This is how ethanol is made naturally, often used for making traditional beer (doro) or wine.
- Raw Materials: Need sugars (like glucose, found in fruits, grains like maize, sugar cane - nzimbe).
- Process: Yeast (mbiriso - a type of microorganism/fungus) is added to a solution of sugar and water.
- Conditions: Keep it warm (around 25-37°C) and without air (anaerobic conditions).
- Reaction: The yeast uses enzymes to break down the sugar into ethanol and carbon dioxide gas.
Glucose ---> Ethanol + Carbon Dioxide - Result: This produces a dilute solution of ethanol (usually only up to about 15% ethanol) because the yeast dies if the ethanol concentration gets too high.
- Step 2: Fractional Distillation (Kusvinura neKupisa):
- To get concentrated ethanol (like the spirit used for cleaning wounds or strong alcohol), you need to separate the ethanol from the water in the fermented mixture.
- Principle: Ethanol boils at a lower temperature (about 78°C) than water (100°C).
- Process: The dilute ethanol solution is heated carefully in special equipment (a fractionating column). The ethanol turns into vapour more easily than water. The ethanol vapour rises up the column, is cooled (condensed) back into liquid, and collected. Water is left behind.
- Result: This process separates the ethanol from most of the water, giving a much more concentrated ethanol solution (up to about 96%).
4. Uses of Ethanol:
Ethanol is a very useful chemical:
- Alcoholic Drinks: It's the alcohol found in beer, wine, spirits (doro rakasimba – in slang: tumbwa).
- Fuel: Can be used as a fuel for cars (mafuta emota), either on its own or mixed with petrol (e.g., "E10" fuel means 10% ethanol). This is common in places like Brazil and sometimes blended locally.
- Solvent: It dissolves many substances that water cannot. Used in perfumes, cosmetics, varnishes, inks, and some medicines.
- Antiseptic / Disinfectant: Used to clean skin before injections or to clean wounds (spirit yekurapa ronda) because it kills germs (bacteria and viruses). Found in hand sanitizers.
- Chemical Feedstock: Used as a starting material to make other important organic chemicals (like ethanoic acid - vinegar).
- Thermometers: Used in some thermometers because it freezes at a very low temperature.
Sub-Topic 2: Global Warming
This is an environmental issue affecting the whole planet (Pasi rese).
Definition of Global Warming:
- Global Warming refers to the long-term heating of Earth's surface observed since the pre-industrial period (roughly before the year 1850) due to human activities.
- Simply put, it means the average temperature of the whole planet (air, oceans, land) is slowly increasing over many years. (panyika pano pane temperature inotariswa kunzi haaa iyi ndo temperature yatongoti ndeye psi rese. Saka temperature iyi yaitarisw kubva kare before 1850 and irikuonekwa kuti irikungoramba ichiwedzera kukura mbichana mbichana zvoreva kuti pasi rino riri kuwedzera kupisa.)
- (Izvi zvinokonzerwa nemabasa arikuitwa nevanhu sechiutsi chavanobudisa kuma industry ma hombe kana chiutsi chinobudiswa ne mota chiya, chinoenda mudenga kumusoro soro.saka zuva parinopisa nyika heat iya inowanzo buda yodzokera ku space, but kana kune chiutsi ichi chakawanda mumhepo, heat iya inobva yatadza kubuda nekuti inenge yabatwa ne chiutsi ichocho chiutsi ichi ndo chinonzi greenhouse gas.)
Causes of Global Warming:
The main cause of global warming is the increase in greenhouse gases in the atmosphere, primarily due to human activities.
- The Greenhouse Effect (How it Works):
- The sun warms the Earth.
- The Earth radiates some heat back towards space.
- Certain gases in the atmosphere (called greenhouse gases) trap some of this outgoing heat, acting like a blanket (gumbeze) or the glass roof of a greenhouse (imba yegirinihausi). This natural effect keeps the Earth warm enough for life.
- The Problem (Enhanced Greenhouse Effect):
- Human activities are releasing too much of these greenhouse gases.
- This makes the "blanket" thicker, trapping more heat than usual, causing the planet's average temperature to rise.
- Main Greenhouse Gases Caused by Humans:
- Carbon Dioxide (CO₂):
- Source: Burning fossil fuels (coal, oil, natural gas) for electricity, industry, and transport (mota, marori, mabhazi, ndege); Deforestation (cutting down and burning forests - kupisa masango) reduces the number of trees that absorb CO₂.
- Methane (CH₄):
- Source: Farming (especially from livestock like cows releasing gas, and rice paddies); decomposition of waste in landfills (marara ari mumadhampi); natural gas leaks.
- Nitrous Oxide (N₂O):
- Source: Use of nitrogen fertilizers in agriculture (manyowa ane nitrogen); industrial processes; burning fossil fuels.
- (Other gases like CFCs also contribute but are being phased out due to ozone layer damage).
- Carbon Dioxide (CO₂):
In short: Burning fuels, cutting forests, certain farming practices, and industrial activities release extra greenhouse gases that trap more heat, leading to global warming.
Let's Summarise: Organic Chemistry & Global Warming
- Ethanol is an alcohol (contains -OH group). Its formula is C₂H₅OH.
- It's produced by fermentation of sugars by yeast (giving dilute ethanol) followed by fractional distillation (to concentrate it).
- Ethanol is used in drinks, fuel, solvents, and as an antiseptic.
- Global Warming is the long-term increase in Earth's average temperature.
- It's mainly caused by the increase in greenhouse gases (like CO₂, Methane) from human activities like burning fossil fuels, deforestation, and farming, which trap too much heat in the atmosphere.
